The diagnostic world of modern medicine has been radically altered by the advent of Optical Coherence Tomography (OCT), a technology that offers a noninvasive window into the microscopic architecture of biological tissues. It is not an X-ray, nor is it a traditional MRI; instead, OCT operates on a principle often compared to ultrasound imaging, but with a fundamental shift in the medium used for probing. Where ultrasound employs sound waves, OCT leverages near-infrared light, allowing it to achieve a resolution that is orders of magnitude finer, often reaching the micrometer level. This capacity for high-resolution, cross-sectional imaging has transformed the management of various diseases, primarily beginning in ophthalmology, but its utility is now expanding across multiple medical disciplines. The ability to visualize tissue morphology in situ and in real-time, without the need for invasive sampling or ionizing radiation, positions OCT as an indispensable tool for both initial diagnosis and the subsequent long-term monitoring of treatment efficacy. The core brilliance of the technology lies in its mechanism for decoding light reflections, a sophisticated process that allows clinicians to perceive layers and microstructures within translucent or semi-opaque materials that would otherwise remain hidden beneath the surface.
OCT is an optical analog of ultrasound imaging that uses low coherence interferometry
At its technical heart, Optical Coherence Tomography relies on a sophisticated optical technique known as low-coherence interferometry. It essentially operates as an optical analog of ultrasound imaging that uses low coherence interferometry to generate its remarkably detailed images. The fundamental setup involves a Michelson-type interferometer, which is a system designed to measure the minute differences in the path length of light. A beam of broad-bandwidth, near-infrared light, often emitted from a super-luminescent diode (SLD), is split into two distinct paths. The first path, known as the reference path, reflects off a fixed mirror with a known distance. The second, or sample path, is directed onto the biological tissue being imaged, such as the retina of the eye. Light that penetrates the tissue is then backscattered or backreflected from the various internal microstructural features and interfaces within the sample. When the light waves from the reference path and the sample path are recombined, they will only produce a measurable interference signal if their path lengths are very nearly equal. By systematically varying the length of the reference path or, in modern systems, analyzing the full spectrum of the reflected light using Fourier transforms, the system can measure the echo time delay and intensity of backscattered light from different depths within the tissue. This depth information is aggregated into columns, or A-scans, which are then compiled as the beam is scanned laterally across the sample to create a two-dimensional B-scan, effectively a cross-sectional “slice” of the tissue.
It captures optical scattering from the tissue to decode spatial details of tissue microstructures
The true diagnostic power of the OCT system stems from its ability to interpret the minute signals it receives, effectively understanding how it captures optical scattering from the tissue to decode spatial details of tissue microstructures. The light that is reflected back is not uniform; different layers and cellular boundaries within the tissue scatter light with varying intensities. For example, in retinal imaging, the differences in light reflectance between the nerve fiber layer (NFL), the ganglion cell layer (GCL), and the underlying inner segment ellipsoid zone (EZ) are distinct. The OCT device’s detector registers these variations in backscattered light intensity, and the built-in software’s segmentation algorithm identifies the borders between these layers. This allows the system to generate a composite image where different tissue types are rendered with distinct levels of brightness—highly reflective structures appear bright, while light-attenuating structures appear darker. This ability to segregate and quantify the thickness of these layers, such as measuring the retinal thickness or the width of the Retinal Ganglion Cell-Inner Plexiform Layer (RGC-IPL), is crucial for monitoring disease progression, particularly in conditions where subtle structural thinning occurs long before functional loss is noticed by the patient.
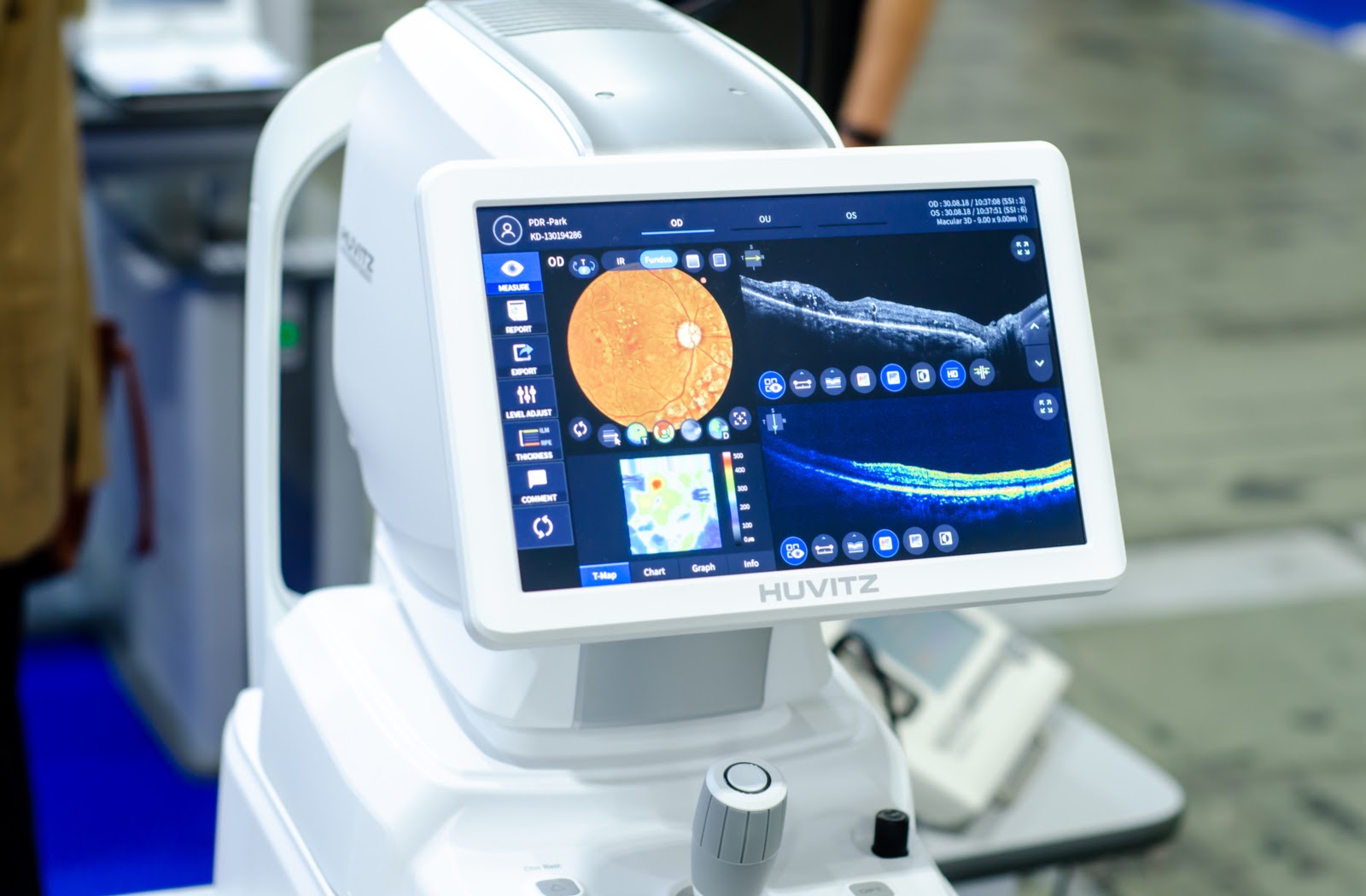
OCT allows eye specialists to examine your eye in layers and to measure the depth of important structures
The primary and most widely recognized application of this technology is in ophthalmology, where OCT allows eye specialists to examine your eye in layers and to measure the depth of important structures. By providing a cross-sectional view of the retina and the optic nerve head, OCT has surpassed older methods in sensitivity and specificity for diagnosing several sight-threatening conditions. For diseases like glaucoma, OCT’s ability to detect the thinning of the retinal nerve fiber layer (RNFL) and changes in the optic nerve head can lead to diagnosis up to six years before the onset of any detectable visual field (VF) loss. Similarly, in diabetic retinopathy and age-related macular degeneration (AMD), OCT is essential for pinpointing the location and extent of retinal edema, the presence of fluid accumulation within or beneath the retinal layers, or the appearance of macular holes or epiretinal membranes. This micrometric precision enables clinicians not only to make an early diagnosis but also to monitor the effectiveness of therapeutic interventions, such as anti-VEGF injections or laser photocoagulation, by quantitatively tracking the changes in fluid volume and retinal structure over time. Furthermore, anterior segment optical coherence tomography (ASOCT) extends this visualization to the front third of the eye, offering high-resolution images of the cornea, anterior chamber, iris, and lens, which is particularly useful in managing angle-closure glaucoma and planning certain ophthalmic surgeries.
OCT has revolutionized the sensitivity and specificity of diagnosis, follow up and response to treatment
Beyond the specialized field of ocular health, the non-invasive, high-resolution nature of the technology has led to a significant expansion of its utility, where OCT has revolutionized the sensitivity and specificity of diagnosis, follow up and response to treatment across other clinical fields. In cardiology, for instance, Intravascular Optical Coherence Tomography (IVOCT), which involves threading a small catheter-based probe into blood vessels, is used to obtain near-microscopic images of the coronary arteries. This allows clinicians to precisely characterize the morphology of atherosclerotic plaque, distinguishing between fibrous, lipidic, and calcific types, and to accurately measure the extent of stent deployment and coverage. This detail is crucial for planning complex percutaneous coronary interventions (PCI) and predicting the risk of future adverse events. The eye itself is also proving to be a window to the heart, with OCT Angiography (OCTA) being used to detect subtle changes in the retinal microvasculature. Current research suggests that these changes, such as reduced capillary densities, may reflect small vessel damage occurring in other organs due to systemic conditions like systemic arterial hypertension or congestive heart failure, establishing retinal microvascular biomarkers as potential indicators for major cardiovascular diseases.
Spectral-domain OCT (SD-OCT) is the most likely form of OCT that you will encounter in a clinic
The technological evolution of OCT has been marked by a relentless pursuit of speed and resolution. The early generation, known as Time-Domain OCT (TD-OCT), was relatively slow, generating images sequentially by physically moving the reference mirror. The transition to the current clinical standard, Spectral-Domain OCT (SD-OCT) is the most likely form of OCT that you will encounter in a clinic. SD-OCT systems abandoned the moving mirror, instead using a spectrometer and a fast-readout camera to simultaneously measure the entire spectrum of the interference signal. The Fourier transform is then used to convert the spectral information into depth information. This parallel processing allows SD-OCT to achieve imaging speeds of tens of thousands of A-scans per second, dramatically reducing motion artifacts and improving image quality. Even newer, and faster, is Swept-Source OCT (SS-OCT), which utilizes a tunable laser as its light source. This innovation allows for even faster scan rates, deeper tissue penetration by using a longer-wavelength light source, and enhanced imaging through media opacities, positioning SS-OCT as the fastest-growing modality in the market due to its advantages in visualizing deeper structures like the choroid and in cases with cataracts.
AI-powered algorithms can automatically analyze OCT images
The future trajectory of OCT technology is fundamentally intertwined with the rise of artificial intelligence (AI). The sheer volume and complexity of the data produced by modern, high-speed OCT scanners make manual, human interpretation an increasing challenge. To address this, AI-powered algorithms can automatically analyze OCT images, dramatically enhancing clinical efficiency and diagnostic accuracy. These advanced computational tools are trained on vast datasets of segmented and labeled OCT scans, enabling them to detect subtle structural changes in the retina and other tissues that might be missed by the human eye. Furthermore, AI is moving beyond mere detection; it is being used to automate image analysis and assist in the segmentation of specific layers, making quantitative measurements more precise and reproducible. Crucially, sophisticated AI models are now being explored to predict disease progression and even personalize treatment plans by extracting subtle biomarkers indicative of systemic health conditions, such as early signs of Alzheimer’s disease or neurological disorders, which manifest as changes in retinal structures.
The total retinal thickness is the measurement of the distance between these two segmentation lines
Interpreting an OCT image, particularly in the retina, relies heavily on quantitative metrics derived from the segmented layers. The software’s ability to precisely identify the boundaries of the various retinal layers allows for reliable and reproducible measurements. A key metric is the retinal thickness, where the total retinal thickness is the measurement of the distance between these two segmentation lines—the line representing the inner surface of the retina and the line defining the outer boundary of the retinal pigment epithelium. Any deviation from the established normative database for a patient’s age and demographic can signify pathology. For instance, retinal thinning may indicate ganglion cell loss characteristic of glaucoma, while retinal thickening might point towards edema caused by diabetic macular swelling. Understanding the appearance of different tissue types based on their light-scattering properties is also vital; for example, in intravascular OCT, calcific plaque appears bright and homogenous with a sharp shadow beneath it due to high light attenuation, while lipidic plaque is darker and has diffuse, “murky” borders.
The development of home-based, handheld, and intraoperative OCT has expanded the possibilities of retinal imaging
Expanding the physical reach of this diagnostic capability is another major area of development. Traditional OCT systems are large, costly, and confined to clinical settings. However, the development of home-based, handheld, and intraoperative OCT has expanded the possibilities of retinal imaging significantly. Handheld OCT devices have proven invaluable in settings where conventional positioning is impossible, such as in the examination of pediatric patients or infants in neonatal intensive care units for conditions like retinopathy of prematurity. Home-based OCT represents a new frontier, allowing patients with chronic conditions, such as wet AMD, to monitor their own disease activity between clinical visits. This portable technology can alert the patient and clinician to a sudden increase in retinal fluid, prompting an earlier intervention and potentially saving vision. Furthermore, intraoperative OCT systems, integrated into surgical microscopes, provide surgeons with real-time, cross-sectional views during delicate ophthalmic procedures, dramatically enhancing the precision and success rate of micro-surgery.
UHR-OCT uses broadband light sources to achieve 3 μm resolution in tissue
While current SD-OCT systems offer a resolution of around 10μm axially, researchers are continually pushing the boundaries of clarity to achieve near-cellular resolution. A specific area of advancement is Ultra High-Resolution OCT (UHR-OCT), where UHR-OCT uses broadband light sources to achieve 3μm resolution in tissue. This significant leap in resolution is accomplished by utilizing light sources with a wider spectral bandwidth, which is inversely proportional to the achievable axial resolution. This enhanced detail allows for a clearer visualization of the finer layers and structures within the retina, such as the external limiting membrane (ELM) and the inner segment ellipsoid zone (EZ), which are crucial for assessing the integrity of the photoreceptor layer. Beyond resolution, new contrast mechanisms like OCT Angiography (OCTA), which visualizes blood flow by detecting the movement of red blood cells, and optoretinography, which studies the functional response of photoreceptors to light, are emerging, transforming OCT from a purely structural imaging modality into a functional one, providing a comprehensive assessment of both tissue structure and physiological activity.
A layered image is created that gives us an incredibly accurate picture of your eye and its structures
In summary, the technology known as Optical Coherence Tomography represents a true breakthrough in non-invasive, high-resolution imaging. It leverages the physics of light interference to effectively perform an “optical biopsy” of living tissue without physical contact or the use of damaging radiation. A layered image is created that gives us an incredibly accurate picture of your eye and its structures, allowing clinicians to detect and monitor minute changes that are undetectable by earlier imaging methods. From revolutionizing the early diagnosis of glaucoma to guiding complex cardiac procedures and extending its reach into neurology and oncology, OCT has become an essential diagnostic cornerstone. The ongoing evolution towards higher speed, deeper penetration via Swept-Source technology, and the integration of artificial intelligence promises to further solidify its role as one of the most powerful diagnostic tools available for visualizing the internal architecture of the human body.
